What is Health Research?
Key message:
Health science or medical science deals with the maintenance or improvement of human health. A patient visits a physician in order to have a disease diagnosed, treated or prevented. Therefore, the physician must have a good medical knowledge. The goal of health research is to develop generalizable knowledge that increases understanding of human biology and can be used to maintain or improve human health.
Health scientists read many scientific articles about studies that have been carried out and conduct
studies themselves. Every research study is designed to answer one or more specific questions. Questions and research design have to fit together.
"The study design affects the types of questions that can be asked, the type of data gathered, the appropriate approach to analysis of the data and the kinds of conclusions that can be drawn from the study. It also impacts the risks associated with the research activity." (https://irb.research.chop.edu/study-design, 07.03.21)
A classification of Health Research
"In principle, medical research is classified into primary and secondary research. While secondary research summarizes available studies in the form of reviews and meta-analyses, the actual studies are performed in primary research. Three main areas are distinguished: basic medical research, clinical research, and epidemiological research. In individual cases, it may be difficult to classify individual studies to one of these three main categories or to the subcategories." (www.aerzteblatt.de/pdf.asp?id=64227, 01.08.19, page 262)

Basic Medical Research
"Basic research is experimental or theoretical work undertaken primarily to acquire new knowledge of the underlying foundations of phenomena and observable facts, without any particular application or use in view." (www.nsf.gov/statistics/randdef/rd-definitions.pdf, 19.06.20, page 3)
Theoretical Basic Medical Research is:
"... the development and improvement of analytical procedures—such as analytical determination of enzymes, markers or genes—, imaging procedures—such as computed tomography or magnetic resonance imaging—, and gene sequencing—such as the link between eye color and specific gene sequences. The development of biometric procedures—such as statistical test procedures, modeling and statistical evaluation strategies—also belongs here." (www.ncbi.nlm.nih.gov/pmc/articles/PMC2689572/, 01.08.23)
Experimental Basic Medical Research is when:
"Scientists conduct laboratory experiments with the building blocks of disease to try to understand how a disease works. They study cells, proteins and DNA from humans and animals, or disease-causing agents such as chemicals, bacteria and viruses. Scientists also study and develop new drugs and treatments in the laboratory. ..." (www.cancer.org.au/content/about_cancer/ebooks/Understanding_clinical_trials_and_research_booklet_July_2018.pdf, 01.08.19)
What is the difference between Clinical Research and Epidemiology?
A clinician tries to maintain or improve the health of an individual; a epidemiologist tries to maintain or improve the health of a community or a population.
"... while the clinician usually focuses on treating and caring for the individual, the epidemiologist focuses on identifying the exposure or source that caused the illness; the number of other persons who may have been similarly exposed; the potential for further spread in the community; and interventions to prevent additional cases or recurrences. ...
To make the proper diagnosis and prescribe appropriate treatment for a patient, the clinician combines medical (scientific) knowledge with experience, clinical judgment, and understanding of the patient. Similarly, the epidemiologist uses ... epidemiologic judgment, and understanding of local conditions in “diagnosing” the health of a community and proposing appropriate ... public health interventions to control and prevent disease in the community." (www.cdc.gov/csels/dsepd/ss1978/lesson1/section1.html, 25.05.20, see under "Specified populations" and "Application")
Clinical Research
What can be done to maintain or improve patient health? Clinical research aims to answer this question.
Clinical Research (Patient Research) is:
"Research conducted on people to better understand, diagnose, prevent and treat diseases. It is usually carried out in a clinical setting such as a hospital or outpatient clinic ..." (www.cancer.org.au/content/about_cancer/ebooks/Understanding_clinical_trials_and_research_booklet_July_2018.pdf, 01.08.19)
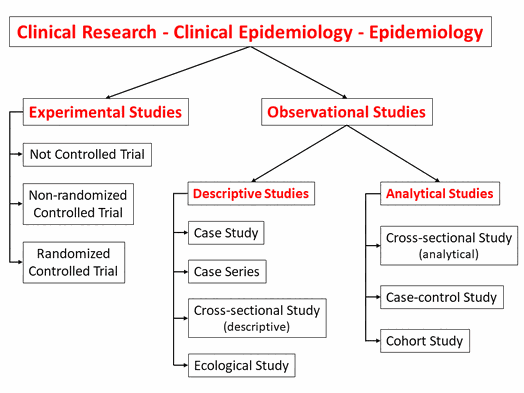
There are two main types of clinical research: interventional studies (clinical trials) and observational studies.
"In a clinical trial, participants receive specific interventions according to the research plan or protocol created by the investigators. These interventions may be medical
products, such as drugs or devices; procedures; or changes to participants' behavior, such as diet. Clinical trials may compare a new medical approach to a standard one that is already available,
to a placebo that contains no active ingredients, or to no intervention. Some clinical trials compare interventions that are already available to each other. ... The investigators try to
determine the safety and efficacy of the intervention by measuring certain outcomes in the participants." (www.clinicaltrials.gov/ct2/about-studies/learn#WhatIs, 06.03.21)
"In contrast, noninterventional clinical studies (NIS) are patient-related observational studies, in which patients are given an individually specified therapy. The responsible physician
specifies the therapy on the basis of the medical diagnosis and the patient's wishes. ... noninterventional
therapy studies include comparison between therapies; however, the treatment is exclusively according to the physician’s discretion. ... Diagnostic studies are another class of observational
studies, in which either the quality of a diagnostic method is compared to an established method (ideally a gold standard) ..." (www.aerzteblatt.de/pdf.asp?id=64227, 01.08.19, page 265)
Epidemiological Research
What can be done to maintain or improve the health of a population? Epidimiological research aims to answer this question.
Epidemiology (Population Research) is:
"The study of how and why diseases occur in groups of people (populations). Scientists working in this field are called epidemiologists. They look for patterns and trends in illness to work out why certain diseases, such as cancer, occur in some people but not in others." (www.cancer.org.au/content/about_cancer/ebooks/Understanding_clinical_trials_and_research_booklet_July_2018.pdf, 01.08.19)
"Are epidemiologists considered medical doctors? No. While epidemiologists study and investigate the causes and sources of diseases in much the same way as medical doctors, they’re not considered actual physicians. Perhaps the biggest reason why is treatment. Generally speaking, epidemiologists do not perform physical examinations on patients, determine diagnoses, or prescribe certain medications." (https://online.regiscollege.edu/blog/learn-career-epidemiologist/, 07.03.21)
Because epidemiologist "study and investigate the causes and sources of diseases in much the same way as medical doctors", it makes no sense to think long and hard about whether a study is a clinical or an epidemiological study. There is even a field of research called clinical epidemiology.
"Clinical epidemiology is the study of the patterns, causes, and effects of health and disease in patient populations and the relationships between exposures or treatments and health
outcomes." (www.ohsu.edu/school-of-medicine/medical-informatics-and-clinical-epidemiology/clinical-epidemiology-research,
07.03.21)
Conclusion: The classification in interventional - observational studies is more important than the differentiation according to patient - patient population - population (the differentiation in clinical and epidemiologic studies).
Some researchers refer to observational
studies as epidemiologic studies. However, this supports the importance of a classification into interventional and observational studies.
"Within primary research there are
observational studies and interventional studies. Observational studies, also called epidemiological studies, are
those where the investigator is not acting upon study participants, but instead observing natural relationships between factors and outcomes. ... Interventional studies, also called experimental
studies, are those where the
researcher intercedes as part of the study design." (Thiese MS. Observational and interventional study design types; an overview. Biochem Med (Zagreb)
2014;24:200)
In fact, it is better to call intervention studies experimental studies because some observational studies also examine interventions, but in these studies the investigator does not choose who of the study participants gets the intervention and who does not.
Here are other explanations for the difference between experimental and observational studies:
"Observational studies observe people in normal settings. Researchers gather information, group volunteers according to broad characteristics, and compare changes over time. ... These studies may help identify new possibilities for clinical trials.
Clinical trials are research studies performed in people that are aimed at evaluating a medical, surgical, or behavioral intervention. They are the primary way that researchers find out if a new treatment, like a new drug or diet or medical device (for example, a pacemaker) is safe and effective in people." (www.nia.nih.gov/health/what-are-clinical-trials-and-studies, 14.10.21)
"A clinical study involves research using human volunteers (also called participants) that is intended to add to medical knowledge. There are two main types of clinical studies: clinical trials (also called interventional studies) and observational studies. ...
In an observational study, investigators assess health outcomes in groups of participants according to a research plan or protocol. Participants may receive interventions (which can include medical products such as drugs or devices) or procedures as part of their routine medical care, but participants are not assigned to specific interventions by the investigator (as in a clinical trial)." (https://clinicaltrials.gov/ct2/about-studies/learn#WhatIs, 14.10.21)
